"If you just focus on the smallest details, you never get the big picture right." – Leroy Hood
The burgeoning landscape of artificial intelligence (AI) in oncology is revealing a remarkable capacity to sharpen our diagnostic abilities, streamline the rigorous testing of new therapies, and even architect novel medicines with unprecedented precision. AI is emerging as a tireless radiologist, an exacting pathologist, an astute clinical trial coordinator, and an innovative molecular chemist, each role promising to refine critical facets of our engagement with cancer. Yet, even the most exquisitely designed drug, conceived through algorithmic intelligence and validated via AI-accelerated trials, must ultimately prove its mettle not in the idealized simplicity of a computational model or a laboratory flask, but within the almost unimaginably complex, dynamic, and unique environment of a human being—a living, breathing, intricately interconnected ecosystem.
For much of its laudable history, our valiant struggle against cancer has been necessarily, and often fruitfully, shaped by a reductionist approach. We've meticulously dissected the disease, breaking it down into its constituent parts: the single rogue gene, the specific mutated signaling pathway, the errant malignant cell, the physically discernible tumor mass. This focused lens has yielded profound insights and undeniably life-saving therapies, from targeted drugs that precisely home in on specific molecular flaws to immunotherapies that brilliantly unleash the body's own defense systems. We owe a great debt, and many lives saved, to this powerful analytical tradition.
However, as our collective knowledge has deepened, it's increasingly clear that cancer is rarely, if ever, merely a disease of isolated genes or individual cells. It is, more accurately, a profound and often cascading systemic disruption of an intricate biological web—the human body itself. This body is not a simple machine, but a dynamic ecosystem, teeming with countless interacting biological processes, constantly influenced by external environmental inputs, shaped by lifestyle choices and internal states, and even modulated by vast, influential microbial communities. All these elements exist in continuous interplay.
The ultimate dream of truly understanding, and thereby comprehensively mastering, cancer lies in moving beyond the intensive study of its individual components in isolation. It beckons us towards a more holistic, integrated view, one that embraces and attempts to model this profound systemic complexity. It is precisely here, at this conceptual frontier, that the powerful confluence of multi-omics technologies, the emerging principles of systems biology, and the formidable analytical and integrative power of artificial intelligence promises a genuine and deeply necessary reboot in our fundamental thinking. This new paradigm offers the potential to see, understand, and ultimately treat the patient as a whole, unique, and evolving ecosystem, not just the isolated disease.
This new systems-level paradigm posits artificial intelligence as the master conductor. Its task is to orchestrate the vast and diverse streams of biological, clinical, environmental, and even behavioral data, aiming to compose a deeply personalized, dynamic "symphony" of individual health, predisposition, disease manifestation, and therapeutic response. We will examine how the integration of multi-omics—the simultaneous, high-resolution analysis of an individual’s genome, epigenome, transcriptome, proteome, metabolome, and microbiome—with rich, real-world data gleaned from wearable sensors, environmental inputs, and even psychosocial factors, is finally paving the way for the kind of Predictive, Preventive, Personalized, and Participatory (4P) medicine. This was a vision articulated decades ago by scientific pioneers like Dr. Leroy Hood, who foresaw a future where medicine would transcend its reactive limitations. This integrated, systems-level understanding, powered by AI, forms the essential foundation upon which a future with far less suffering from cancer—and perhaps one day, the ability to largely prevent or control this ancient malady—might eventually be built.
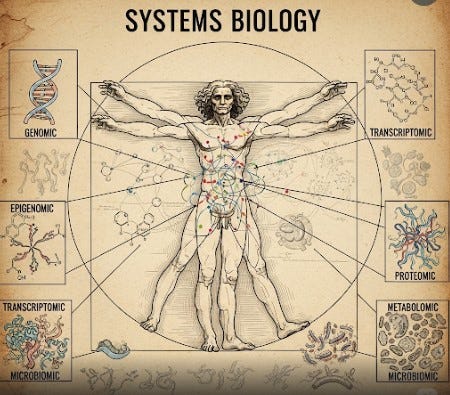
The Unfolding Map of Ourselves: An Introduction to Multi-omics
The Human Genome Project provided humanity with the first comprehensive "blueprint" of human life, igniting hopes for an era where complex diseases could be understood and conquered by deciphering their genetic roots. While genomics has revolutionized oncology, delivering powerful targeted therapies, it also unveiled a more complex truth: the genome alone tells only a fraction of the intricate biological story.
Cancer, we now comprehend, arises from a complex, often decades-long, and highly individualized interplay of inherited genetic predispositions, acquired somatic mutations, dynamic epigenetic modifications, diverse environmental exposures, myriad lifestyle choices, and unpredictable stochastic events. All these factors manifest their influence through dynamic changes across multiple, interconnected molecular layers within our cells, tissues, and entire bodies. To truly comprehend this intricate biological dance, we must look beyond the static DNA blueprint to the array of dynamic "omes" that collectively define our unique biological state at any given moment. This is the rapidly expanding, data-rich domain of multi-omics.
Imagine the genome as the master architectural blueprint for a vast and incredibly complex city.
Genomics studies this primary blueprint—the complete DNA sequence of an individual, identifying inherited variations and acquired somatic mutations that might predispose to disease or influence drug response.
Epigenomics explores the annotations and modifications on that blueprint—chemical tags like DNA methylation that do not alter the underlying DNA sequence but act like master switches, determining which genes are turned "on" or "off."
Transcriptomics focuses on the "active messages" being sent out from the genome—the myriad RNA molecules transcribed from currently active genes. It provides a dynamic, real-time snapshot of ongoing cellular activity.
Proteomics studies the actual protein machinery of the cell—the enzymes, receptors, and structural components that execute the vast majority of cellular functions. Proteins are the cell's true workhorses.
Metabolomics analyzes the chemical fingerprints of ongoing cellular processes—the diverse array of small molecules that are consumed, produced, and transformed during cellular activity. This provides a real-time readout of a cell's metabolic state, often dramatically altered in cancer.
Microbiome research investigates the trillions of symbiotic microbial partners that inhabit our bodies. These vast and diverse communities profoundly influence our metabolism, immune system, and response to therapies.
Each of these distinct "-omic" layers provides a valuable, yet incomplete, perspective. The true, revelatory understanding emerges only when we can effectively integrate these distinct layers, observing how changes in one "ome" cascade through and influence the others, ultimately creating the complex phenotype of health or disease. This intricate integration of multi-layered, high-dimensional biological data is a daunting analytical task, one for which artificial intelligence is uniquely and powerfully equipped.
Systems Biology: Seeing the Forest and the Trees (and the Mycorrhizal Networks Within)
If multi-omics provides an ever-more-detailed inventory of the molecular components within our biological "city," then systems biology seeks to understand how these myriad components interact dynamically to form a functioning, coherent, and adaptive whole. It strives to map how information flows through networks, how resources are allocated, and how disruptions in one localized area can propagate and affect distant parts of the entire organismal system.
Systems biology moves beyond a purely reductionist view, embracing the concept of intricate biological networks and the fascinating, often counterintuitive, emergent properties that arise from them. These are characteristics of a complex system that manifest from the specific interactions of its individual parts and often cannot be predicted or fully understood by studying those parts in isolation. The whole, in systems biology, is indeed often greater, and certainly different, than the simple sum of its parts.
Consider the ecological analogy. A skilled ecologist doesn't just study individual species; they strive to understand the entire forest ecosystem: how the layered canopy influences light, how animals disperse seeds, and how vast underground fungal networks connect root systems, facilitating nutrient exchange and communication. Disease in this ecological context is often seen not merely as a problem confined to a single afflicted species, but as a potential failure or imbalance within the broader ecosystem.
Similarly, systems biology views the human body, and cancer arising and progressing within it, as an extraordinarily intricate, multi-scale network of networks. Genes interact in complex regulatory circuits. Proteins form elaborate signaling cascades. Metabolic processes are tightly interconnected. Our immune system is in constant, nuanced communication with virtually every cell.
Cancer often arises when these intricate networks become dysregulated, when critical communication pathways break down, or when rogue cellular elements hijack the system's resources for their own uncontrolled proliferation. Understanding these complex network dynamics—how they are "wired" in health, how they become rewired in disease, and how they respond to perturbations—is absolutely fundamental to developing more effective, precise, and ultimately less toxic interventions that can restore systemic balance. The challenge, of course, lies in the almost unimaginable complexity of these biological systems. This is precisely where the unique computational power, advanced pattern-recognition capabilities, and integrative analytical strengths of artificial intelligence become indispensable tools.
Leroy Hood's Vision: The Arrival of 4P Medicine
Long before artificial intelligence and multi-omics technologies matured, the visionary biologist Dr. Leroy Hood championed a new, proactive, and deeply personalized paradigm for healthcare: 4P Medicine—Predictive, Preventive, Personalized, and Participatory. This prescient framework anticipated a fundamental shift from reactive treatment of established disease to a proactive, wellness-oriented approach.
Let's briefly revisit the essence of these four foundational pillars:
Predictive: The ability to forecast an individual's likelihood of developing specific diseases based on their unique germline genetic makeup, evolving multi-omic biomarker profiles, and relevant environmental exposures and lifestyle factors, often long before overt symptoms appear.
Preventive: The capacity to translate these individualized predictions into proactive, tailored interventions, including lifestyle modifications, nutritional strategies, chemoprevention, or enhanced surveillance schedules designed to prevent disease or intercept it at its most nascent stage.
Personalized: The meticulous tailoring of both preventive strategies and therapeutic interventions to the unique biological, clinical, psychosocial, and contextual makeup of each individual patient, moving away from "one-size-fits-all" approaches.
Participatory: Empowering patients to become active, informed, and engaged partners in their own health journey, fostering a collaborative relationship with healthcare providers centered around shared decision-making.
For many years, Hood's 4P vision remained more of an aspiration due to the lack of robust, scalable tools to generate and interpret the necessary multi-omic and real-world data. Today, however, the powerful and rapidly accelerating confluence of high-throughput multi-omics technologies, the ubiquitous proliferation of digital health tools and sophisticated wearable sensors, and the exponentially growing analytical power of artificial intelligence are finally providing the necessary infrastructure. These advancements are beginning to bring 4P medicine from theory into practical, impactful clinical application, particularly in a complex, heterogeneous, and dynamically evolving disease like cancer.
Expanding the Data Universe: Beyond the Clinic Walls
A true systems understanding of human health and the complex origins of cancer requires us to look far beyond the data traditionally collected within clinic visits or hospital stays. Our internal biological ecosystem is in constant, dynamic dialogue with our external environment, daily behaviors, dietary choices, sleep patterns, stress levels, and social interactions. Artificial intelligence's remarkable ability to integrate vastly heterogeneous data types is key to capturing this broader, more holistic picture.
Wearables as Personal Sentinels: Capturing Life in Motion The astonishing proliferation and increasing sophistication of consumer wearable devices (e.g., Apple Watch, Fitbit, Oura Ring) and clinical-grade continuous glucose monitors (CGMs) are generating an unprecedented, continuous stream of real-time physiological and behavioral data from individuals as they navigate their everyday lives. These devices track heart rate, heart rate variability, sleep, physical activity, body temperature, respiratory rate, and blood oxygen saturation. CGMs provide dynamic insight into glucose metabolism. For cancer patients undergoing treatment or high-risk individuals, this continuous data, when intelligently analyzed by AI, could offer invaluable early warnings of physiological decline, emerging treatment-related side effects, signs of infection, or even the faintest harbingers of disease recurrence or development. This moves us from infrequent, static snapshots to a far more dynamic, longitudinal, and deeply personalized understanding of an individual's evolving health status.
The Environment and Lifestyle: Critical Inputs to the Systemic Equation Our individual biology does not exist in an isolated vacuum. It is constantly shaped by the air we breathe, the water we drink, the food we consume, our physical environment, toxins, and the nature of our social connections. AI offers the powerful potential to integrate data on these diverse, often difficult-to-quantify, external factors:
Environmental Exposures (The Exposome): Information on local air and water quality, historical or ongoing exposure to carcinogens (e.g., radon, asbestos), and occupational hazards can be correlated with individual health records and multi-omic profiles. This helps identify and quantify environmental risk factors or understand how exposures interact with genetic predisposition.
The Profound Impact of Lifestyle and Psychosocial Factors: Insights from "Blue Zones" powerfully underscore the profound impact of lifestyle and social context on health. Factors like chronic psychosocial stress, social isolation, loneliness, and disrupted sleep patterns are increasingly recognized as having detrimental systemic effects on chronic inflammation, immune function, and metabolic health, potentially influencing cancer development and progression. AI can help quantify these variables—perhaps through privacy-preserving analysis of social media text, validated digital survey data, or sensor-derived proxies for stress responses—and integrate them into comprehensive risk assessment and treatment planning models.
By systematically incorporating these diverse, real-world data streams, artificial intelligence can build a far richer, more contextualized, and more dynamically responsive model of each individual's unique "ecosystem." This allows for a deeper, more nuanced understanding of how external factors modulate internal -omic layers and contribute to the delicate balance between sustained health and the initiation or progression of cancer.
AI: The Master Integrator and Pattern Weaver
The sheer volume, velocity, and variety of data generated by multi-omics technologies, continuous wearable sensors, digitized environmental records, and detailed lifestyle tracking present a monumental analytical challenge. We are talking about potentially thousands, or even millions, of distinct data points per individual, collected longitudinally, spanning disparate modalities from DNA sequences to daily activity patterns. Making coherent, actionable sense of this multi-scale, multi-modal data deluge is far beyond the capacity of traditional statistical interpretation by human researchers alone.
This is precisely where artificial intelligence, particularly sophisticated machine learning and deep learning algorithms, becomes the indispensable master integrator and pattern weaver. AI algorithms are uniquely suited to:
Integrate Diverse and Disparate Data Types: AI can effectively handle and harmonize data in its myriad forms—structured data (genomic sequences, lab values), unstructured data (clinician notes, patient narratives), time-series data (wearable sensors), and complex imaging data. Advanced techniques explicitly model the complex, often non-linear, relationships between these different data entities, creating a unified analytical framework.
Identify Novel Biomarkers and Complex Risk Signatures: By analyzing deeply integrated datasets across large populations, AI can identify novel, multi-modal biomarkers or complex, multi-feature risk signatures that can predict disease onset, progression, or treatment response with significantly greater accuracy than any single data type. For example, an AI model might discover that a specific combination of a genetic variant, an epigenetic mark, a shift in gut microbiome, and a pattern of nocturnal heart rate variability is highly predictive of an aggressive cancer subtype.
Model Complex Biological Interactions and System Dynamics: AI can learn and model the intricate, non-linear interactions and feedback loops between genes, proteins, metabolites, microbial communities, lifestyle factors, and environmental exposures that collectively underpin health and drive disease processes. This moves beyond statistical correlations to fostering a more mechanistic, systems-level understanding of biological dynamics, allowing for "what if" simulations of interventions.
Create "Digital Twins" or Dynamic Personalized Health Models: The ultimate goal is to use AI to create a dynamic, computable, and highly personalized mathematical model of an individual's unique biology—a "digital twin." This virtual model, continuously updated, could then simulate the effects of different interventions specifically for that individual, predicting likely trajectories of health or disease and helping identify optimal, highly personalized strategies in silico before application to the patient.
Generate Actionable Clinical Insights: Crucially, AI's role is not just to find complex patterns. The true value lies in its ability to translate these intricate patterns and model-based predictions into clear, understandable, and actionable insights that can directly inform clinical decision-making for more precise prediction, more effective prevention, and the delivery of truly personalized therapeutic strategies. It's about transforming a cacophony of disparate data into a coherent, clinically useful "symphony of the self."
The Promise for Cancer Care: A Systems Reboot in Action
The thoughtful and ethical application of this AI-driven, multi-omic, systems biology approach to cancer care holds the promise of a profound and far-reaching paradigm shift, one that will touch, and hopefully dramatically improve, every aspect of a patient's journey:
True Early Prediction & Proactive Prevention: Instead of relying predominantly on broad, often imprecise, population-level risk factors and age-based screening guidelines, imagine being able to identify individuals at exceptionally high, precisely quantifiable risk for developing specific cancers years or even decades before the disease would typically manifest clinically. This identification could be based on a continuously updated, deeply personalized, integrated risk score derived from their unique and evolving combination of germline genetics, acquired somatic mutations, epigenetic changes, dynamic microbiome profile, subtle physiological drifts detected by wearables, and environmental exposures. Such early and precise prediction would enable the implementation of highly targeted, truly personalized preventive interventions.
Personalized Interception of Incipient Disease: For those individuals where cancer has just begun its almost imperceptible molecular stirrings, often long before it forms a macroscopic tumor detectable by conventional imaging techniques, a sophisticated systems approach, fueled by AI and multi-omics, could potentially identify the unique constellation of systemic imbalances and early molecular derangements that are driving this incipient pathological process. Interventions could then be exquisitely personalized, aiming to restore homeostasis and eliminate these earliest errant cells.
Holistic and Adaptive Treatment Strategies for Established Disease: When cancer does unfortunately develop and is diagnosed, treatment decisions could be informed by a comprehensive, dynamic systems view of the patient's entire biological and personal ecosystem, not just the characteristics of the tumor itself. AI could help select the optimal combination and sequencing of therapies—not just targeted drugs, chemotherapies, and immunotherapies, but also interventions aimed at favorably modulating the tumor microenvironment, bolstering the patient's systemic immune system, or proactively mitigating anticipated treatment side effects. Treatment would thus become a far more dynamic, responsive, and adaptive process. AI could constantly integrate new incoming data to help clinicians adjust therapeutic strategies in near real-time, maximizing efficacy while continuously minimizing toxicity and preserving or enhancing the patient's overall quality of life.
Enhanced Quality of Life and Comprehensive Survivorship Care: By understanding the broader systemic impact of cancer and its often-grueling treatments on an individual's entire being—their physical functioning, their metabolic health, their immune status, their psychological well-being, their social interactions—AI could help design and deliver more personalized, proactive, and effective supportive care plans. This could involve optimizing nutrition, promoting physical activity, and more effectively addressing critical psychosocial needs. This holistic approach aims to significantly improve the overall quality of life for patients during their active treatment and throughout their often lengthy and complex survivorship journey.
Conclusion: The Dawning of Systemic Wisdom and a New Dialogue with Our Health
We stand at the dawn of an era where our understanding of cancer, and indeed of human health and disease more broadly, is undergoing a profound, almost Copernican, transition: from a primary focus on individual, often isolated, biological components and linear causal pathways to a more holistic and integrative appreciation of the entire, intricate, interconnected, and dynamic system that is a human being. The powerful convergence of multi-omics technologies, wearable sensors and digital health tools, digitized environmental data, and detailed lifestyle factors, all orchestrated, analyzed, and interpreted by the sophisticated pattern-recognition and modeling capabilities of artificial intelligence, offers the first genuine possibility of achieving the deeply holistic, truly personalized, and proactively preventive vision of 4P medicine that pioneers like Lee Hood so clearly foresaw decades ago.
This represents far more than just an accumulation of ever-larger quantities of disparate data; it is about striving towards, and beginning to achieve, a new level of systemic wisdom. It signifies a fundamental reboot in our scientific and clinical approach, moving us progressively from a predominantly reactive battle against established, often advanced, disease towards a proactive, lifelong cultivation of resilient health within the unique, evolving ecosystem of each individual patient. This journey requires not only brilliant algorithms and powerful computers but also a profound and sustained shift in our collective thinking, a commitment to seeing and understanding the whole person in all their complexity, and a renewed, unwavering dedication to the humanistic core of medicine. This dedication ensures that these remarkable new tools serve not merely as technological marvels, but as instruments to deepen our understanding, enhance our capacity to care, and empower our patients in the most comprehensive and compassionate sense.
This dedication to the humanistic core, to the well-being of both patient and practitioner, brings us to a critical juncture. The very tools that promise to unravel cancer's systemic complexity, that aid in designing new generations of precisely targeted medicines, and that sharpen our diagnostic acuity to detect disease at its faintest whisper, also hold the potential for a more immediate, deeply personal kind of reboot—a reboot for clinicians themselves, addressing the pervasive, often soul-crushing, burdens of current medical practice. Having explored how artificial intelligence can help us see cancer earlier, design smarter clinical trials, conceive novel drugs, and understand the patient as a dynamic ecosystem, a crucial and intensely practical question now takes center stage: How can these intelligent systems help us, the oncologists and dedicated care teams on the front lines, navigate our demanding days not just more effectively, but perhaps, dare we hope, with a renewed sense of joy and purpose in our work?
Can these sophisticated algorithms, which detect, predict, and design with such astonishing power, also lift the often numbing weight of administrative duties that consumes so much of our precious time? Can they untether us from the perceived tyranny of keyboards and the endless checkboxes of electronic medical records, thereby restoring our primary, undivided focus to the patient before us, to their families, to the nuanced, irreplaceable human interactions that are the very soul of medicine? Can artificial intelligence, in short, help us reclaim the precious, irreplaceable gift of time—time to think, time to connect, time to heal—allowing us to rediscover and re-engage with the profound human connections and the deep intellectual challenges that drew us to this demanding yet deeply rewarding field in the first place?
These are not idle questions; they touch the heart of our professional lives, the sustainability of our calling, and the very nature of compassionate care. We must now turn our gaze from the expansive frontiers of biological discovery and systemic understanding to the pressing, palpable realities of daily clinical practice. We will explore how artificial intelligence might indeed help us reboot the practice of oncology itself, examining what our daily lives could become if we were substantially freed from the administrative and documentation burdens that currently weigh so heavily upon us, allowing us to more fully embrace and enact the art and science of medicine—and the profound human privilege of caring—that we so passionately chose.




AI integration once you have a diagnosis is such a promising and exciting prospect and I’m confident this will be achieved sooner rather than later. However, while I love it, I’m struggling a bit with the concept of practical implementation of the multi-omics and 4P proactive prevention strategies.
This sounds like it could be promising and offer hope to many. Studying side effects or possible side effects of the suggested treatments would be imperative